In a mixture of gases each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The Ideal Gas Law - The relationship between volume pressure temperature and quantity of a gas including definition of gas density.

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy
The partial pressure is the pressure that each gas would exert if it alone occupied the volume of the mixture at the same temperature.

. To Learn expressions on Daltons law of partial pressure Examples Videos with FAQs. Volumetric gas fraction converts trivially to partial pressure ratio following Daltons law of partial pressures. Where P B 0 is the vapor pressure of pure liquid component B.
An example where Henrys law is at play is in the depth. Daltons law of partial pressures - for calculating partial pressures. More often than not you will see the partial pressure of a gas being expresses in terms of its mole fraction.
Also in the 1800s he was the first scientist to explain the behavior of atoms in terms of the measurement of weight. ColorblueP_gas chi_gas xx P_mixture. In any given mixture each gas component exerts its own pressure which is called the partial pressure independent of other gases.
Pi XiPT Root-mean-square speed of gas molecules. The ideal gas law also called the general gas equation is the equation of state of a hypothetical ideal gasIt is a good approximation of the behavior of many gases under many conditions although it has several limitations. Mathematically it can be written as.
Raoults law ˈ r ɑː uː l z law is a relation of physical chemistry with implications in thermodynamicsProposed by French chemist François-Marie Raoult in 1887 it states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component liquid or solid multiplied by its mole fraction in the mixture. If youre seeing this message it means were having trouble loading external resources on our website. Gay-Lussacs law also referred to as Amontons law citation needed states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant.
It was formulated by the English chemist William Henry who studied the topic in the early 19th century. Partial pressure blending at constant temperature is computationally simple. This empirical law was observed by John Dalton in 1801 and published in 1802.
It encompasses the study of the conditions under which fluids are at rest in stable equilibrium as opposed to fluid dynamics the. It is caused by the anesthetic effect of certain gases at high pressure. Developed by chemist and physicist John Dalton who first advanced the concept of chemical elements being made up of atoms 9 X Research source Daltons Law states that the total pressure of a gas mixture is the sum of the pressures of each of the gases in the mixture.
It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyles law Charless law Avogadros law and Gay-Lussacs law. This empirical relation was stated by the English chemist John Dalton in 1801. Made by the Tula Arms Plant Тульский Оружейный Завод Tulskiy Oruzheynyy Zavod in Russia it is.
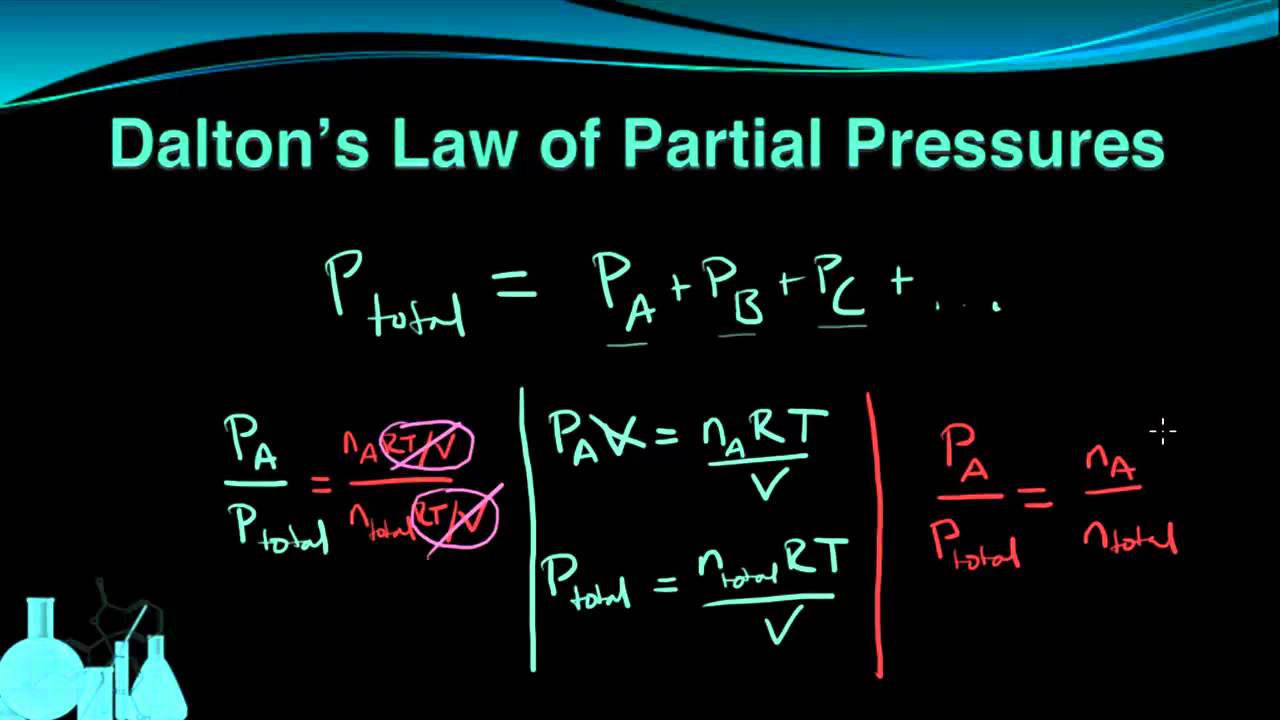
Daltons Law The pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases. The partial pressure of a gas is a measure of. In 1803 he revealed the concept of Daltons Law of Partial Pressures.
In physical chemistry Henrys law is a gas law that states that the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid. If the partial pressure of hydrogen is 1 atm find the mole fraction of oxygen in the mixture. Definition of partial pressure and using Daltons law of partial pressures.
The Greek word νάρκωσις narkōsis the act of making numb is derived from νάρκη narkē numbness torpor a. The APS underwater assault rifle APS stands for Avtomat Podvodny Spetsialnyy Автомат Подводный Специальный or Special Underwater Assault Rifle is an underwater firearm designed by the Soviet Union in the early 1970s. Definite Composition A compound is composed of two or more elements chemically combined in a defined ratio by weight.
Gay-Lussac is recognized for the Pressure Law which established that the pressures of an. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. Daltons law also called Daltons law of partial pressures states that in a mixture of non-reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
Solved Examples on Daltons Law of Partial Pressure Example 1. P B x B. P tot P i P 1 P 2 P 3.
Daltons law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases. Now we will apply Daltons law of partial pressures. Given P hydrogen 1 atm P total 15 atm.
P i the. PV nRT where P is pressure V is volume n is the number of moles R is the gas constant and T is temperature. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 15 atm on the walls of its container.
Narcosis while diving also known as nitrogen narcosis inert gas narcosis raptures of the deep Martini effect is a reversible alteration in consciousness that occurs while diving at depth. P an2 V2 V - nb nRT Definition of heat capacity where s. The proportionality factor is called Henrys law constant.
P tot the total pressure. It is a special case of the ideal gas law. The term partial pressure is used when we have a mixture of two or several gases in the same volume and it expresses the pressure that is caused by each of the induvidual gases in the mixture.
The total pressure of the gas mixture is the sum of the partial pressure of the component gases. The gases present in the container are chemically inert. Understand Daltons Law of Partial Pressures.
The key to this problem is the fact that each component of a gaseous mixture will contribute to the total pressure exerted by the mixture proportionally to the number of molecules in has in the mixture. For calculating the pressure of a nonideal gas. This law tells us that the total pressure P total of the solution placed in a container is the sum of partial pressures of its respective components.
P B P B 0 x B. Similarly partial pressure of B will be. Total and Partial Pressure - Daltons Law of Partial Pressures - How to calculate total pressure and partial pressures for.
Visit BYJUS for more content. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture Daltons Law. Partial pressure blending is commonly used for breathing gases for diving.
The accuracy required for this application can be achieved by using a. Partial Pressure- Partial Pressure is defined as a container filled with more than one gas each gas exerts pressure. Daltons law is related to the ideal gas laws.
It was adopted in 1975. The partial pressure is calculated easily by following the Daltons Law of Partial Pressures whereas the vapor pressure is calculated by applying the Raoults Law. Urms 3RT M05 Van der waals equation.
This lets us find the most appropriate writer for any type of assignment. The pressure of any gas within the container is called its partial pressure. The law used to find partial pressure assumes the temperature of the system is constant and the gas behaves as an ideal gas following the ideal gas law.
Fluid statics or hydrostatics is the branch of fluid mechanics that studies the condition of the equilibrium of a floating body and submerged body fluids at hydrostatic equilibrium and the pressure in a fluid or exerted by a fluid on an immersed body.

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
0 Comments